Orbitals and quantum numbers are essential concepts in the study of atomic structure and chemical bonding in chemistry.
What is an Orbital?
An orbital is a mathematical function that describes the probability of finding an electron in a particular region of space around an atom. In other words, an orbital is a three-dimensional region in space where the electron is most likely to be found. Each orbital can accommodate a maximum of two electrons, and electrons in the same orbital must have opposite spins due to the Pauli exclusion principle. The shape, size, and orientation of the orbitals are determined by the four quantum numbers.
Quantum Numbers
The four quantum numbers are a set of numbers that are used to describe the unique quantum state of each electron in an atom. The first quantum number is the principal quantum number (n), which determines the energy level or shell of the electron. The second quantum number is the azimuthal quantum number (l), which determines the shape of the orbital and the subshell in which the electron is located. The third quantum number is the magnetic quantum number (m), which determines the orientation of the orbital in space. The fourth quantum number is the spin quantum number (s), which describes the intrinsic angular momentum, or spin, of the electron.
Together, the orbitals and quantum numbers provide a way to describe the electronic structure of an atom and explain its chemical properties. The understanding of these concepts is crucial for predicting and explaining chemical reactions and chemical bonding between atoms.
Here is a brief explanation of each quantum number:
Principal Quantum Number (n): This quantum number describes the energy level or shell of an electron in an atom. It can take any positive integer value starting from 1, and determines the distance of the electron from the nucleus. As the value of n increases, the energy of the electron increases and its distance from the nucleus also increases.
Azimuthal Quantum Number (l): This quantum number describes the shape of the orbital that the electron occupies. It can take any integer value from 0 to n-1, and determines the subshell in which the electron is located. The possible subshells are labelled as s, p, d, f, etc. For example, if n=2, l can be 0 or 1, corresponding to the subshells 2s and 2p.
Magnetic Quantum Number (m): This quantum number describes the orientation of the orbital in space. It can take any integer value from -l to +l, including zero. The number of orbitals in a subshell is equal to 2l+1. For example, in the p subshell (l=1), there are three orbitals with m=-1, 0, and +1, which correspond to the three perpendicular directions in space.
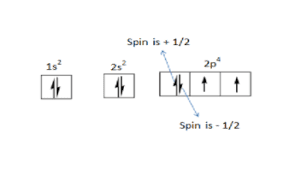
Spin Quantum Number (s): This quantum number describes the intrinsic angular momentum, or spin, of the electron. It can take the value of +1/2 or -1/2, which corresponds to the direction of the
electron's spin. Electrons in the same orbital must have opposite spins due to the Pauli exclusion principle.
Together, these four quantum numbers specify the unique quantum state of each electron in an atom, and they provide a way to describe the electronic structure of an atom.